
See here for all our coverage of the COVID-19 pandemic.
Lateral flow device (LFD) tests for COVID-19 have become a common feature in our lives. Many parents are being asked to use them to test their school aged children twice weekly with the aim to both reduce the spread of the disease in our schools and reduce the number of school days that children miss (you can read more about the use of LFD tests in schools here).
But how do LFD tests differ from the more familiar PCR (polymerase chain reaction) tests we have been using since the beginning of the pandemic? And what impact could they make to controlling the spread of the disease?
Taking the test
The NHS lateral flow device test kit (Image: tommoh29
The experience of taking an LFD test is similar to taking a PCR test at home. You follow the instructions for using a swab to take a sample from the back of your throat and up your nose. But rather than send the sample off to a lab to be processed, which can take anything from 1 to 3 days to get a result, you instead transfer the sample to the lateral flow device and wait just 30 minutes to get the result at home.
The quicker result is possible because the LFD test is using a different marker to the PCR test for the disease. The PCR test looks for particular genes from the virus. The small amount of genetic material in a sample is repeatedly amplified in the lab, with the amount of DNA in the sample doubling with each cycle of the process. The more cycles required for a sample to reach a detectable threshold for the virus, the lower the viral load for the person tested. The number of cycles required to detect the COVID-19, called the cycle threshold (Ct), then provides a measure of how much virus the person had (their viral load) at the time of the test. It's thought the higher the viral load (ie the lower the cycle threshold) the more infectious the person is.
LFD tests detect the virus by seeing if the sample triggers a visible change in the test strip using the labelled antibodies in the device. Any virus in a sample binds with the antibodies in the strip, and these bound virus particles then accumulate on the test zone of the strip, triggering the appearance of the visible line indicating the person has tested positive for COVID-19.
Breaking chains of infection
Not only will an individual person's viral load vary over the course of their infection, there is also a great deal of variation between people in how this progresses. But some time after you are exposed to the virus your viral load increases sharply, usually reaching the high levels associated with infectiousness (which is thought to be when Ct is less than 30) a few days after exposure. You are likely to start being infectious a day or two before developing symptoms (if you are going to develop them).
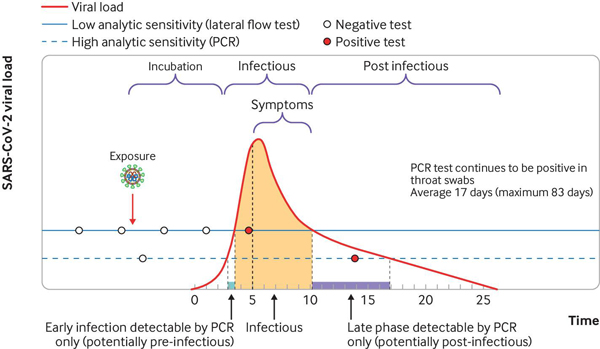
An example of a person's infection trajectory with their viral load (measured by the Ct value) given over time (the red line). The infectious period is shaded, which begins in the days before any symptoms might develop. Although LFD tests are less sensitive to viral load (shown by the solid horizontal line) than PCR tests (the dashed horizontal line), they are likely to give a positive result during the infectious period, particularly when testing is regular and frequent. Because PCR tests are more sensitive, it is possible for them to give a positive result after the infectious period, when LFDs would no longer detect the virus. (Image from Alex Crozier et al. BMJ 2021;372:bmj.n208, used with permission.)
One of the reasons the SARS outbreaks in 2003 did not become a pandemic was that people only became infectious after they developed symptoms, meaning they could be isolated before they became infectious. But, because people with COVID-19 can infect others before they develop symptoms, or even if they don't go on to develop symptoms, this policy isn't enough to contain the spread of COVID-19. This difference contributed significantly to COVID-19 becoming a pandemic.
"On average, it takes two or three days after infection begins before it's detectable on any test," says Elizabeth Fearon, an epidemiologist from the London School of Hygiene and Tropical Medicine. Fearon is leading a project investigating Test, Trace and Isolate (TTI) strategies, work funded by a UKRI/NIHR Covid-19 rapid response grant. "Then the viral load rises very steeply, peaking just before you start to develop symptoms. The viral load stays high for about a week, then tails off over a long time."
When the viral load is high, which is when a person is thought to be the most infectious, both LFD and PCR tests are most sensitive and more likely to detect cases of COVID-19. But PCR tests can detect lower levels of viral load than LFD tests, so you are more likely to get a positive PCR test later in your infection, when the viral load is lower, than a positive LFD test result.
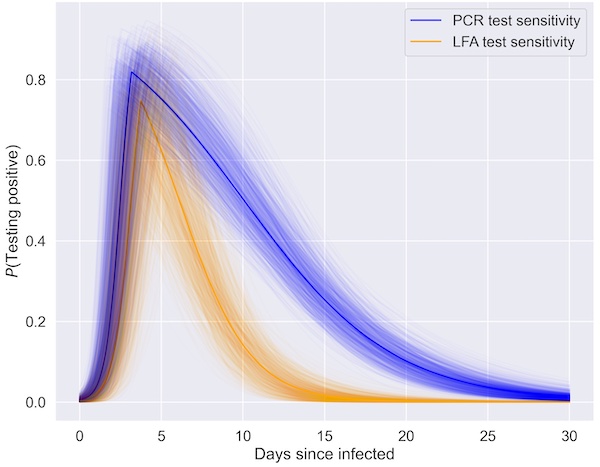
Probability of testing positive with a lateral flow test (yellow) and a PCR test (blue) over the course of an infection with COVID-19. The spread in both of these give an indication of the uncertainty in these estimates (Image: Elizabeth Fearon and Martyn Fyles, used with permission)
"The PCR tests can detect lower levels of viral load than LFD tests," says Fearon. "This means that PCR tests are better at picking up cases later in the infection when the viral load is lower, so LFD tests will miss some of these." But as it is thought that people aren't so infectious later in the infection, when the viral load starts to tail off, these missed cases may not result in as much spreading of the disease. "Also detecting people with low viral loads can sometimes be a bad thing, for example if it meant making recovered individuals [who may still have low viral loads] isolate when they are not infectious," says Martyn Fyles, a researcher on the TTI project and a PhD student at the University of Manchester.
One of the impacts that the use of LFD tests may have comes from their comparatively low cost and the fact that they can be carried out by people in their own homes, without the need for skilled staff to conduct the tests and analyse the results. This means that LFD tests can be used for widespread regular schedules of testing in the population, for example to monitor the spread of COVID-19 in schools, as is currently done.
Widespread regular testing of people without symptoms means possibly detecting people who are infected with COVID-19 who would not have been tested through the PCR testing programme (as PCR tests in the UK are usually only available to people with symptoms). These people might have asymptomatic COVID-19 (around a third of people with COVID-19 do not develop any symptoms, or only develop very mild symptoms) or their infection might be detected in the days before they go on to develop symptoms. Detecting these cases, and the infectected person then isolating, could break chains of transmission that would not have been broken by use of the PCR tests alone.
"Regular testing which is easy to use plays an important role in controlling COVID-19, because it allows outbreaks to be detected early, particularly when many people are not showing symptoms," says Deirdre Hollingsworth, a member of the JUNIPER consortium and one of the contributors on the TTI project.
What about false results?
No test is 100% accurate. For any test, a small number of people will receive a negative test result when they are infected – a false negative. This is more likely for an LFD test than for a PCR test, but if we're thinking about the impact of these tests for general use, the speed at which you could test yourself again could overcome this to some extent. "[LFD tests] have the advantage of speed and lower costs, enabling their more widespread, repeated, and immediate use," says Fearon. "As discussed above, LFDs are also much less likely to give a false negative result when a person is at their most infectious."
For any test there's also a small possibility that people receive a positive test result when they are not infected – a false positive. It has been estimated that the probability that an LFD test will return a positive result when the person is truly negative for infection is 1 in 1000 or even lower; the probability that this happens twice is 1 in 1,000,000 (because 1/1000 x 1/1000 = 1/1000000), assuming this probability is independent each time you take the test. "Mitigating the impact of false positive test results is why confirmatory PCR testing is currently used for people testing positive on an LFD test, but this practice incurs an additional delay for the PCR test to be booked and the results returned," says Fearon.
The probability that the person receiving a positive test result is actually positive (called the positive predictive value) depends on the prevalence of infection in the community; when prevalence reduces, the probability that a person receives a false positive result increases. (You can read a full explanation of how prevalence impacts on the positive predictive values a test here.)
One worry about LFD tests is that if people have to isolate on a positive LFD test result, then the rate of false positives means that many people will end up isolating unnecessarily. But the alternative, as Fearon explains, could be worse. "All infection controls like lockdowns and contact tracing involve uninfected people isolating," says Fearon. "There is a personal cost to people isolating. We don't want to dismiss that and they should be financially and socially supported. But we need to remember that the counterfactual to [a small number of people isolating] as a result of false positives isn't that nobody has to isolate, it is that everybody has to isolate as the epidemic will get out of control and we have to lock down."
Modelling Test, Trace and Isolate strategies
Fyles' PhD has been on modelling the transmission of Hepatitis C on network structures and the role of contact tracing, but naturally in the pandemic he is now using these approaches for studying the spread of COVID-19. "Our work uses a branching process model to study the transmission of the disease," says Fyles. "The branching process produces a random, tree-like structure of infections. Contact tracing then spreads along the tree-like structure, and attempts to control the spread of the disease."
The model uses a complex social network of connections within and between households, with the distribution of the size of households matching the results of the 2019 ONS survey and the pattern of social contacts building on the previous CoMix and Polymod studies. The model is seeded with a number of infections, and then run forward one day at a time, modelling both the spread of infections within households, called local epidemics, and between households. An infected person infects those they are in contact with with a probability based on how long they have been infected, related to viral load, with this probability peaking at around 5 days into their infection.
Fearon and Fyles can then vary the parameters of the model to simulate the effect of changes in interventions, such as modifying the practice of the Test, Trace and Isolate system. In particular, they have been looking at the impact that daily testing with LFD tests could have in reducing the amount of quarantine time for contacts of infected people, while also reducing the spread of disease.
Daily contact testing
Fearon's project was asked by the Scientific Pandemic Influenza Modelling Group (SPI-M), which contributes to government advice, to investigate the use of LFD tests in the Test, Trace and Isolate system. The current practice is that if someone develops symptoms or tests positive, they and others in their household isolate for 10 days, and NHS Test and Trace try to reach as many of the infected person's contacts as they can. Their contacts might have become exposed and infected themselves, so these contacts are asked to quarantine (a term used for those isolating who don't know if they are infected) as soon as possible before they have had the chance to infect anyone else. These contacts are asked to quarantine for 10 days from the day they last made contact with the infected person. The people who live with these contacts do not have to quarantine unless the contact goes on to develop symptoms or test positive.
Fearon and Fyles' work suggests an alternative which involves less isolation. Instead of quarantining, people in contact with an infected person could take a lateral flow test every day, and only isolate if they tested positive for COVID-19. Fearon and Fyles used their model to investigate different scenarios of daily contact testing: in some scenarios all contacts (including within the infected person's household) took daily LFD tests and didn't quarantine, in some scenarios household contacts also quarantined, and in both cases contacts had to take daily LFD tests for varying lengths of time. Their work suggests that daily contact testing with LFD tests in all these scenarios could be as effective as the current quarantine strategies.
One particular question they were asked to investigate was the impact requiring a positive PCR test after a positive LFD test to initiate contact tracing might have on the spread of the disease. Their research showed that waiting for a confirmation result before starting contact tracing was less effective than beginning contact tracing immediately on getting a positive LFD test result.
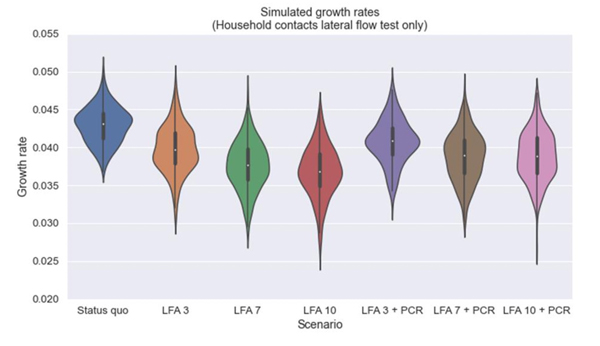
Comparing the impact of daily contact testing on the growth rate of the disease compared to the current TTI strategies. In these scenarios there is no quarantine for either the infected person's household contacts or their out-of-household contacts. Instead these contacts take daily LFD tests for 3 days, 7 days, 10 days, with and without the infected person taking a confirmation PCR tests. All these daily contact testing strategies were more effective than the status quo, and the requirement to take a PCR test before starting contact tracing reduced the effectiveness. For comparability the modelling assumed 100% adherence to the status quo isolation and quarantine and to the daily contact testing, but these assumptions would not be realistic in practice. (Image from SPI-M consensus statement, 3 March 2021, used with permission)
This modelling gives an idea of the impact such changes to the Test, Trace and Isolate system could have, both on reducing the growth of the epidemic and reducing the burden of quarantining unnecessarily for contacts of cases. But the modelling also shows how dependent the success is on the capacity and the willingness of the community to follow any strategy. "Adherence to such testing strategies and isolation policies determines their effectiveness and needs careful consideration," says Fearon. "Any changes will alter the findings greatly, possibly to the point of negating any positive impact of daily contact testing." Trialling the results of such modelling in practice is important, says Fearon. And this is now being done as part of a large contact testing study involving over 40,000 people.
As we move out of lockdown and try to find a way to live with COVID-19, finding long-term strategies for containing the disease must be acceptable to everyone in order to be effective. Provided the population is enabled and supported to take up testing and isolation, daily testing with LFD tests could provide a more acceptable strategy to strict quarantine for contacts of positive cases, while still being effective at stopping the spread.
About this article
Elizabeth Fearon is assistant professor of epidemiology at the London School of Hygiene and Tropical Medicine. Fearon is leading a project investigating Test, Trace and Isolate (TTI) strategies, work funded by a UKRI/NIHR Covid-19 rapid response grant.
Martyn Fyles is a PhD student at the University of Manchester and a researcher on the TTI project.
Deirdre Hollingsworth is professor and senior team leader at the Big Data Institute at the University of Oxford. She is also a member of the JUNIPER consortium and one of the contributors on the TTI project.
Rachel Thomas is Editor of Plus.
This article was produced as part of our collaboration with JUNIPER, the Joint UNIversity Pandemic and Epidemic Response modelling consortium. JUNIPER comprises academics from the universities of Cambridge, Warwick, Bristol, Exeter, Oxford, Manchester, and Lancaster, who are using a range of mathematical and statistical techniques to address pressing question about the control of COVID-19. You can see more content produced with JUNIPER here.
