Career interview: Medical statistician

Rob at the MCA offices in London
It always looked like Rob Hemmings was going to have a career in mathematics - Rob says he was a "scary child", explaining that "while my brother was playing with Action Men I was playing with numbers!" But now he find that pure mathematics holds little interest for him - he is more concerned with what maths can do in the real world.
He tells Plus about his career as a medical statistician, first designing and analysing trials of new products for a big pharmaceutical company, now working as poacher-turned-gamekeeper for the UK Medicines Control Agency.
Just maths for me and everything else was dull

University of Nottingham.
[Image used by permission of the University of Nottingham]
Reminiscing about his school days, Rob tells a story about a maths test a teacher gave his class in middle school. "Quite a few of us got 40 out of 40. But I remember they weighted our scores by age, and as I was one of the youngest in the year, I got the highest marks! That was probably a big turning point. From then on it was just maths for me and everything else at school was dull!"
Rob attended Shelley High School, a comprehensive in Yorkshire, where he took Maths, Further Maths, German, Economics and General Studies for his A-levels. That resulted in A's in Maths, Further Maths and General Studies and B's in German and Economics, which got him a place at the University of Nottingham to do a Maths degree. He chose Nottingham because "it had a good reputation, it was a nice town, and it gave me an offer! I didn't really want to come to London, and I did look around Warwick, York, a couple of other places, but I just liked Nottingham. The campus was excellent."

University of Nottingham.
[Image used by permission of the University of Nottingham]
So the choice of maths for a degree subject was straightforward, but Rob found he didn't enjoy the theoretical side of maths as much as he had expected. He explains that he's "not someone who's interested at all in the technical side of maths. I'm only interested in what it can do, in a practical setting, what you can use it for - which I'm sure is why I moved towards statistics." One particular course appealed to him more than the rest - Medical Statistics, which he took in his third year. He says the lecturer made the subject sound fascinating, showing just how important statistics was in modern medicine. The course directly prompted his next move after graduation from Nottingham in 1995 - a one-year MSc at Southampton University in Statistics with Applications in Medicine. Still the practicalities were what interested Rob the most. He says the course "contained a little bit too much theory for my liking, but I guess it was all necessary and it hasn't seemed to do me any harm!"
Less theory, more practice

[Image DHD Photo Gallery]
Given his interests in the practical side of things, Rob found it easy to decide on his next step. He took a job at Zeneca (now AstraZeneca), a big pharmaceutical company with offices in Macclesfield, about fifteen miles south of Manchester. He loved working there "because there were about 7,000 people who worked for Zeneca. It was the big employer of the area. You were leaving university and going straight to a company where there were lots of other people who were just leaving university. There were always people your own age, loads of new starters all the time, so you could share a house easily - it was just like staying at university but getting paid for it!".
Rob's first job for Zeneca was as a statistician working in the Phase 1 unit. He explains that in the development of new medicines, the first thing that happens is that researchers discover a particular molecule with chemical properties that suggest it might have a therapeutic effect. They test the molecule on a huge collection of standard targets - chemicals and synthesised cells in test tubes - to see which sort of cells it is likely to affect. It's a hit-and-miss business. "Far fewer than 1% of molecules that somebody gets excited about in the laboratory actually get through to being licensed."
After the drug has passed through various (very tightly regulated) stages of animal testing, it is time for the first humans to be exposed. This stage is called Phase 1. Rob's first job was on "first-into-man" trials - which means exactly what it sounds like. "The very first trial is usually a rising dose study. You give a small group of volunteers very small doses of a particular molecule and see what happens to them." Then when the researchers (and the volunteers!) are happy that the drug is not obviously poisonous - bear in mind that all medicines are poisons to some extent! - and they've identified a range of doses from nothing to the sort of dose that gives unacceptable toxicity - "unacceptable" varies depending on whether the drug is to cure a headache or to treat cancer - the researchers try to identify a range of doses that might be "efficacious" - in other words, that work. They investigate the "pharmacokinetics" and "pharmacodynamics" of the drug - what the body does to the drug (how quickly it gets in, where it goes, how quickly it gets out again), and what the drug does to the body (if it raises blood pressure, relieves pain, lowers the heart rate, and so on).
Phase 1 trials throw up some challenging problems for a medical statistician. Rob explains that it is necessary to use the principles of randomisation, for example "to ensure you get no bias by the medic picking a particular patient - 'he looks small and scrawny, we'd better give him a low dose'!". Because there are so few patients involved, a statistician will have to use clever designs, which subsequently require a lot of technical analysis. Rob's job was "everything from designing to analysing to reporting the trials and helping interpret them."
In the course of his year working in the Phase 1 unit, Rob worked on about 10 or 12 different compounds, some, but not all, of which went on to Phase 2 trials, and might eventually get licensed for use in the UK. The need for a licensed medicine to cover the costs associated with unsuccessful attempts to develop other medicines is one of the main drivers for setting the eventual market prices of licensed treatments. In Phase 1 it is less serious, from the company's point of view, to let a bad candidate through than to miss a good candidate, as there are many more hurdles for an experimental medicine to clear; but "at the same time, if you can identify the bad ones and not throw good money after bad in Phase 2 trials then you've done your job."
After Phase 1 - Phase 2 and Phase 3
Next Rob moved to a group developing a particular anti-cancer drug. The team designed, analysed and reported on Phase 2 and Phase 3 trials. "We were looking for efficacy in colorectal, pancreatic, ovarian, and non-small-cell lung cancers. I think that was my favourite time while I was there. I found designing trials more interesting than analysing trials - it was more creative, and involved more problem-solving." His least favourite aspect of his job was SAS programming. SAS stands for "Statistical Analysis System" and it's a universal data analysis tool used in the pharmaceutical industry. "We had a course on it for the Masters, then used it frequently at Zeneca, and the best thing about my current job is that I hardly ever touch it. I hated SAS programming!

All the data
[Image freeimages.co.uk]
"A clinical trial report can be volumes and volumes. You have maybe 50 or 100 pages of typed description of the trial and interpretation of the trial, and than anything from another 100 to thousands of pages of data. Anything that you measure you've got to report. As part of our training as statisticians we had to produce all these tables and listings, which was bearable in Phase 1 because the trials weren't that big, but fortunately by the time I had gone to Phase 3 there were dedicated SAS programmers to do that sort of thing. A Phase 3 team would be about a dozen people - a project leader, a couple of medics, a couple of statisticians, a couple of CRA's [Clinical Research Associates] - the people who do the dirty work of setting up and running the trials - then maybe a couple of SAS programmers and a medical writer."
Rob's fondest memories of that time are of the travel. Many of the big drug trials for that particular product took place in the US, and he was back and forth across the Atlantic talking at meetings with the doctors involved in treating the trial patients.
After the year working on the anti-cancer drug, Rob spent his third year with Zeneca working on products to treat asthma and schizophrenia. His next move took him to his current post - Statistical Assessor for the Medicines Control Agency in London. Although he still loved his job at Zeneca, he had itchy feet, and when he saw the ad for the post, he thought he might as well give it a try - even though he didn't have as much experience as was stipulated. "I thought they could only say no, stuck my CV in the post, was surprised to get an interview, and even more surprised to get the job!"
Poacher-turned-gamekeeper

The MCA offices in London
The Medicines Control Agency is part of the Department of Health. It has trading fund status which means it's selfsufficient. Rob says "we run like a small company. I call myself a civil servant when it suits me - but I don't really feel like one".
The job of the MCA is to regulate medicines and to protect and promote public health within the UK. It is split into seven divisions, including the Licensing Division, which is where Rob works. It is their job to give out drug licences for products they are convinced meet the required standards for "quality, safety and efficacy", demonstrated through good manufacturing practice, good clinical practice, good laboratory practice and the clinical trials run by the companies and submitted to the MCA for assessment. Rob's colleagues in the Licensing Division have varied backgrounds in laboratories, medicine, academia, and commercial work for drug companies.
They've all been approved
[Image DHD Photo Gallery]
Rob says that what he likes best about his job is that no two days are the same. As a statistical assessor, he must read the reports describing clinical trials of medicines submitted by drugs companies - and help decide whether they deserve to be licensed. He needs to decide whether the trials described were designed, analysed and interpreted appropriately. As he says, every application that comes in claims that their drug is safe and efficacious - otherwise the company wouldn't be applying!
As a statistician, Rob mainly concentrates on efficacy, and works closely with people with medical training. Primarily people with medical training consider the question of whether a product is safe, and pharmacists and toxicologists consider quality. Once Rob has come to a conclusion as to whether a drug is efficacious, he writes a report which in turn becomes part of a larger report. For most products, Rob and his colleagues decide whether to license the product; for others, particularly for new medicines, they consult the Committee on Safety of Medicines, an external committee of experts in all relevant fields. Rob looks at approximately 30 products a year, the majority of which are eventually approved and granted licences.
The one who sits at the end of the line
Rob says bluntly that he was "bored of the theory. You can get quite complicated trial designs and analyses. I didn't want to be the one sat at the pharmaceutical company working out how to do this analysis, what my sums of squares was, and what matrix to use, etc. I certainly didn't want to be the one working out how to program it in computer language. I'm much more interested in being the one at the end of the line deciding whether other people have done their analyses properly! What I try to do is apply statistical principles in a logical way to drug trials, in order to say whether a drug's been proven beyond reasonable doubt to be efficacious."
Although the job doesn't have any of the travel Rob enjoyed so much in his previous work, he does collaborate with his counterparts in other European countries. Arranging Europe-wide licences for drugs that have been licensed for use in Britain is very challenging. "Getting a medic and a statistician to agree whether efficacy has been shown or not can be a challenge, reaching agreement between the MCA assessors and the CSM can also be a challenge. Sitting as representatives of the UK amongst representatives from France, Germany, Denmark, all of the EU member states, and trying to agree on whether a product should have a licence - that's where the fun really starts!"
It's important to Rob that he does a job that has practical implications. "I've been in situations where the UK is the only country in Europe with a problem about a particular drug being granted a licence, because of a statistical anomaly or a problem with the data, and I've got to convince representatives from every other country that we shouldn't give it a licence. At the end of the day it comes down to a subjective decision of a number of people like myself - and those infinitely more qualified! That's the best bit of the job. I think the main criterion for doing my job properly is to be able to form and communicate opinions - not something that mathematicians are noted for being good at, but something I really enjoy."
Not long ago, there was no regulation governing which drugs that reached the market. Drug regulation was essentially born of the Thalidomide tragedy. When Plus asked if Rob ever worried about playing a small part in another such nightmare, he explained that if he makes a mistake, it means that an inefficacious product gets to the market, rather than an unsafe one. However, he is confident that the rigorous testing regime makes this very unlikely indeed.
"Rather than being worried about it I look at it the other way - I'm quite proud that we might say yes to a drug that might make a difference. If I meet people and they ask what I do and I say 'medical statistician' it doesn't sound very interesting at all. I usually say I'm a fighter pilot! But if you actually explain what the job is and what the end product of the decision-making is, it makes it sound rather grand. I enjoy the fact that I can think I'm making a difference."
About this article
Helen Joyce is editor of Plus.
You can find out more about the Medicines Control Agency and its work at www.mca.gov.uk/home.htm.
As you can see from the graph below, statisticians are playing an ever-larger role in the pharmaceutical industry. For more on careers in statistics, you can visit The Royal Statistical Society's career pages.
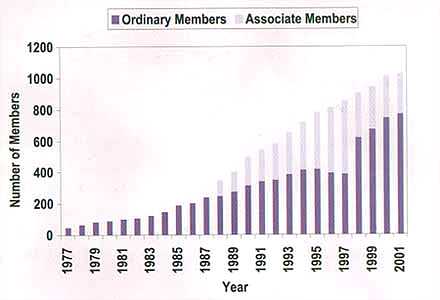
Membership of PSI (Statisticians in the Pharmaceutical Industry)