The roll-out of COVID-19 vaccines in the UK is going well, so it seems there are grounds for optimism. But what can we really say about where the vaccines have got us so far and where we are likely to be when the rollout is complete?
See here for all our coverage of the COVID-19 pandemic.
Recent work by a team from the JUNIPER modelling consortium provides some insight. It suggests that vaccination on its own is highly unlikely to eradicate COVID-19. However, it's got a key role to play in getting us out of the lockdown. Here caution is crucial, the work suggests. The slower the pace of relaxing the rules, the flatter the curves of future infection waves.
The team consists of epidemiologists from the University of Warwick: Sam Moore, Edward Hill, Michael Tildesley, Louise Dyson, and Matt Keeling. Most of the team contribute to the Scientific Pandemic Influenza Modelling Group (SPI-M) and Keeling is also a member of the Joint Council for Vaccination and Immunisation.
How good are the vaccines deployed in the UK proving to be?
There's a lot of uncertainty surrounding almost every aspect of the pandemic, and this applies to vaccines too. When they were first approved, clinical trials had shown that the Pfizer/BioNTech and Oxford/AstraZeneca were safe and effective enough to be put to use, but precise figures on just how well they work can only be obtained once large numbers are vaccinated.
The data that has been collected since the roll-out started has provided a clearer picture. As Keeling reported in a research talk hosted by the Isaac Newton Institute last week, latest estimates say that the vaccines offer different levels of protection against different aspects of infection.
Ideally a vaccine would stop people from even becoming infected, but it's also possible that a vaccine merely stops you from developing symptoms. The crucial difference here is that a vaccine that only stops symptoms wouldn't block the onward transmission of the disease. The infection would still be able to circulate in the population and those who can't (or won't) be vaccinated wouldn't be protected.
Although there's still not enough data to be sure, the figures Keeling reported in his talk give ground for optimism. The vaccines appear to be between 50% and 80% effective at stopping people from even catching COVID-19, and therefore in blocking transmission, and around 90% effective in stopping them from developing severe symptoms.
The effect of the vaccines also increases from the first to second dose. In Keeling's talk he assumed that the level of protection against disease was 70% two weeks after the first dose, rising to 88% two weeks after the second dose.
These percentages are high but they're not maximal, and that's one reason why vaccination alone probably won't eradicate COVID-19. "Because the shield [provided by the vaccines to the individual] is partial, it can be overcome by increases in the level of infection in the general population," says Keeling. In other words, some people will become infected with COVID-19 despite being vaccinated, and the more infection there is in the general population, the more we are likely to see such vaccine failures.
Another thing we couldn't possibly know before the vaccines were rolled out was just how many people would agree to be vaccinated. And here the numbers exceed expectations, in a good way: so far uptake in those over 60 is at 95%.
Are we getting it right?
The work of the JUNIPER team helped to inform how the vaccines are being rolled out, both in terms of the order in which groups of the population are being vaccinated, and in terms of the gap that is left between the two jabs. With both aspects there are different approaches that could have been taken: prioritising people with many social contacts over the old and vulnerable, for example, or aiming for fewer people getting both jabs quickly, rather than more people getting the first jab quickly.
The JUNIPER team began looking at vaccines strategies last summer (see here), using mathematical models that predict the number of cases, hospital admissions and deaths we are likely to see under various assumptions. There's always uncertainty of course, so the outputs of such models can't be regarded as sure-fire predictions. Instead they allow us to explore possible scenarios in an if-this-then-that kind of way. In the case of the vaccine roll-out, prioritising the old and vulnerable persistently turned out as the optimal strategy in the modelling, even as the underlying assumptions were being varied. This is basically the strategy that has been adopted in the UK. (See here to find out more about mathematical models used in epidemiology.)
As far as the spacing between doses is concerned, recent work by Hill and Keeling suggests that prioritising the first dose — giving as many people as possible one jab, rather than fewer people both jabs — is generally the best strategy for the vaccines and vaccine capacity we have. The policy adopted in the UK depended on a number of practical considerations — not least the fact that the Oxford/AstraZeneca vaccine is more effective after a 12-week delay between jabs. Hill and Keeling's work shows that the strategy also has benefits in terms of reducing the number of deaths.
Where are we going?
Mathematical models can also be used to explore what might happen in the future. The model employed by the JUNIPER team is detailed, taking into account, for example, the age structure of the population, geography, the B1.1.7 variant, and who has been vaccinated to date. It's one of the models that also helps scientists calculate the value of the famous reproduction number R every week. "Overall [the model] is supposed to give a fairly complete picture of what has been happening to date, and therefore we hope can give reasonable predictions of what is going to happen going forward in time, although there is always uncertainty with human behaviour," says Keeling.
The first question you might ask is what vaccination does to R — and the answer, according to the model, is that vaccination on its own is not going to get R to below 1. "We're in a situation where vaccination can have a significant impact on R, but in these simulations it's not sufficient to drive R below 1. This is really saying that although the vaccine is doing well to control the disease, it's never going to put us in a situation where we can eradicate it without other controls," says Keeling.
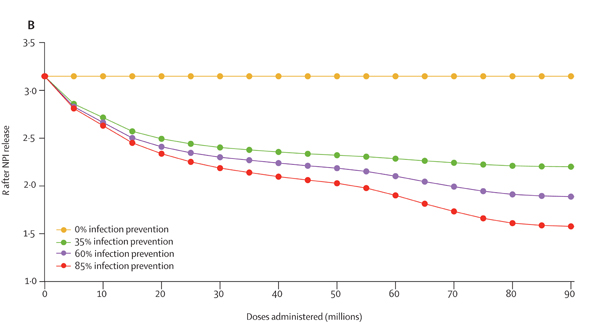
This figure shows how the value of R is estimated to change as more doses of the vaccine are administered in the population, in the absence of any restrictions. The four different lines correspond to four different assumptions on how much infection the vaccines prevent, as shown in the key. Figure from this paper by the JUNIPER Team, which has appeared in The Lancet.
The team also simulated the effects of relaxing the rules, under various assumptions. "The first thing we [asked] is, what if we completely relaxed everything in April?" says Keeling. "We end up with an absolutely enormous outbreak leading to a large number of deaths and hospitalisations. Even after four months of vaccination, if we stop all controls we get a disastrous result."
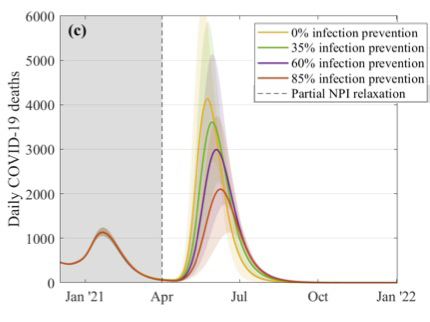
This figure explores what might happen to the daily number of COVID-19 deaths if restrictions were suddenly lifted in April. The different curves correspond to different assumptions about the efficacy of the vaccines at preventing infection, as shown in the key. These simulations assume that 2.5 million doses of vaccines are being given a week, that the efficacy is 70% after the first dose and 88% after the second dose, and that uptake is 95% in the over 80s, 85% in the 50 to 79 age group, and 75% in 18 to 49 age group. Figure from this paper by the JUNIPER Team, which has appeared in The Lancet.
Even if we wait until December and then lift all measures, the model suggests, we can still end up with a large and sustained outbreak, unless the vaccines are very good at preventing infection. "Any abrupt change is always likely to precipitate some form of future outbreak," says Keeling.
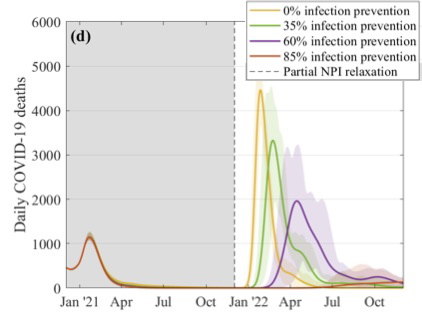
This figure explores what might happen to the daily number of COVID-19 deaths if restrictions were suddenly lifted in December. The different curves correspond to different assumptions about the efficacy of the vaccines at preventing infection, as shown in the key. These simulations assume that 2.5 million doses of vaccines are being given a week, that the efficacy is 70% after the first dose and 88% after the second dose, and that uptake is 95% in the over 80s, 85% in the 50 to 79 age group, and 75% in 18 to 49 age group. Figure from this paper by the JUNIPER Team, which has appeared in The Lancet.
The government's roadmap for coming out of lockdown doesn't involve a sudden relaxation of rules, of course, but takes things step by step. The JUNIPER team also looked at such gradual relaxation strategies and found that, according to their model, the slower the pace the smaller the outbreaks. "A five month relaxation still gives a noticeable peak in deaths, even if [the vaccines offer] an 85% protection against infection," says Keeling. As we move to slower and slower relaxation, the outbreaks become less threatening.
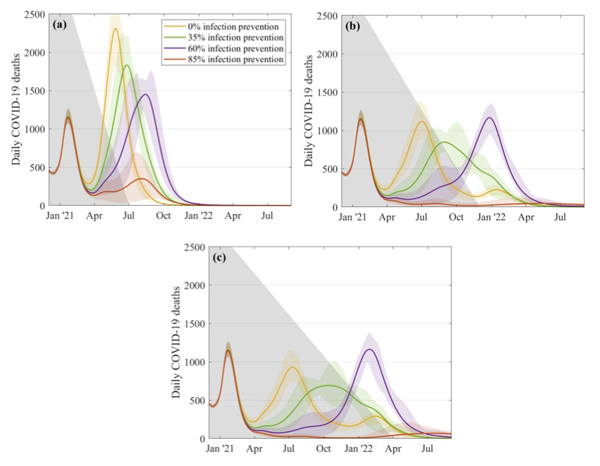
This figure explores what might happen to the daily number of COVID-19 deaths if restrictions are relaxed over a 5 month period (top left), a 10 month period (top right) and a 14 month period (bottom). The different curves correspond to different assumptions about the efficacy of the vaccines at preventing infection, as shown in the key. These simulations assume that 2.5 million doses of vaccines are being given a week, that the efficacy is 70% after the first dose and 88% after the second dose, and that uptake is 95% in the over 80s, 85% in the 50 to 79 age group, and 75% in 18 to 49 age group. Figure from this paper by the JUNIPER Team, which has appeared in The Lancet.
"The message from all this is that we need to be cautions," says Keeling. "Slower relaxation always works better and higher levels of infection blocking are needed to be able to control the outbreak."
The figures above were produced in January and February with the information that was available then. It's important to remember that things are developing very fast with new data on the efficacy of the vaccines becoming available all the time. "Everything I tell you this week is likely to be tweaked and modified next week," Keeling says. However the general message from the simulations remains the same when the model is run on the latest information — although there is more optimism about how well the vaccine might prevent infection.
In summary, then, the modelling shows that vaccination isn't a silver bullet, but does have a key role to play in helping us come out of lockdown. What exactly is going to happen, the model suggests, will depend critically on exactly how good the vaccines are at blocking infection, how slowly restrictions are released, and how many people agree to be vaccinated. In the longer term, waning immunity and new variants that can evade the vaccine will change this simple picture — allowing further future waves unless we have repeated booster vaccine programmes.
About this article
This article is based on research talk by Matt Keeling hosted by the Isaac Newton Institute and a recent paper by Keeling and Sam Moore, Edward Hill, Michael Tildesley, Louise Dyson.
Sam Moore is a postdoctoral research associate who has been working on vaccination modelling for Covid-19 after joining the SBIDER group within the University of Warwick at the start of the pandemic last year.
Edward Hill is a postdoctoral research associate in the SBIDER group at the University of Warwick. He has been a participant in the Scientific Pandemic Influenza Modelling Group (SPI-M) since April 2020.
Michael Tildesley is a Reader at the University of Warwick. He has been a participant in the SPI-M modelling group since April 2020.
Louise Dyson is an Associate Professor in Epidemiology at the University of Warwick. She has participated in the SPI-M modelling group since April 2020.
Matt Keeling is a professor at the University of Warwick, and holds a joint position in Mathematics and Life Sciences. He is the current director of the Zeeman Institute for Systems Biology and Infectious Disease Epidemiology Research (SBIDER). He has been part of the SPI-M modelling group since 2009 and is a member of the Joint Council for Vaccination and Immunisation.
Marianne Freiberger is Editor of Plus.
This article was produced as part of our collaborations with JUNIPER, the Joint UNIversity Pandemic and Epidemic Response modelling consortium, and the Isaac Newton Institute for Mathematical Sciences (INI).
JUNIPER comprises academics from the universities of Cambridge, Warwick, Bristol, Exeter, Oxford, Manchester, and Lancaster, who are using a range of mathematical and statistical techniques to address pressing question about the control of COVID-19. You can see more content produced with JUNIPER here.
The INI is an international research centre and our neighbour here on the University of Cambridge's maths campus. It attracts leading mathematical scientists from all over the world, and is open to all. Visit www.newton.ac.uk to find out more.

