
Mixing it up
It's time for a coffee break. You make the coffee, pour in the milk and give it a stir. But rather than mixing in, the milk forms into a creamy ribbon circling the cup. Your coffee break has been thwarted by anti-diffusive milk.
Thankfully this scenario hasn't been experimentally observed in real-world coffees, but anti-diffusion was certainly discussed during the coffee breaks and workshops of the six-month research programme, Anti-diffusive dynamics: from sub-cellular to astrophysical scales (ADI) at the Isaac Newton Institute for Mathematical Sciences in Cambridge.

Usually if we add milk to a cup of coffee the milk will gradually spread, turning the dark coffee a lighter brown, an example of diffusion. But in some instances alongside diffusion there is another competing process – anti-diffusion – that instead sharpens the boundaries between the different substances. A striking example of anti-diffusion can be seen in the distinct bands in the atmosphere of Jupiter, and it is thought to play an important role here in the atmosphere and oceans on Earth too.
Anti-diffusion also arises in other contexts with very different underlying processes, such as in plasma fusion reactors, the structures that develop in biological matter, and even the flocking of animals or birds. But despite this, anti-diffusion is not well understood physically or mathematically, which is why the ADI programme brought together experts from all these different areas to learn from each other.
Layers and staircases
The process of diffusion has been studied for centuries and is well understood mathematically. The mathematician Joseph Fourier developed a mathematical description for the way heat spreads through metal in the early nineteenth century. This heat equation is now known as the diffusion equation as it can be used to describe a process whereby something that starts highly concentrated in one area spreads out to areas where the concentration is lower. This diffusion happens through an underlying random process, such as the random motion and interaction of particles in a mixture. (You can read more about diffusion in our brief introduction.)
Anti-diffusion, however, is a relatively new concept. It was first proposed theoretically in the 1970s and finally observed in detail in experiments in the 1990s. "Normally when you stir a fluid you just add to diffusion. We stir the milk in our coffee and we mix things up faster," says David Hughes, a fluid dynamicist from the University of Leeds and one of the organisers of the ADI programme. But counter-intuitively there are experiments that show this isn't always the case.
Imagine we have a container of salty water, where the heavier saltier water is at the bottom, and the lighter, less-salty water is at the top. If we drew a graph of how the density of the water increases with depth, we'd usually see a gentle curve.

But strange things can happen when we start to stir the fluid. "It depends on how you stir it, but the initial gentle curve can develop quite a strong staircase, where there are regions where the density is [well] mixed up followed by an abrupt jump in density to another region."
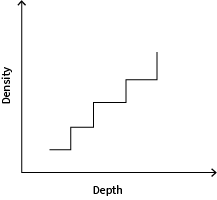
Staircases are an example of the structures that can develop when anti-diffusion is present. These abrupt jumps in density are also seen outside the lab, for example, in our oceans.
There are two diffusive processes – temperature and saltiness – at play in our oceans, but they happen at very different rates. Heat diffuses about a hundred times faster than salt, and the interaction of these two diffusive processes is called double diffusion. Researchers think that it might be this large difference in the scale of the two processes involved that can lead to the anti-diffusion observed in our oceans.
"There are two interesting situations that occur, both of which lead to staircases in the temperature, salinity and density," says Hughes. "The first, which occurs in warm seas such as the Mediterranean, is when warm salty water overlies colder, fresher water." The thermal gradient of this situation is stabilising: the hot water is above the cold water as we'd expect. (Heat normally rises as hot water is normally less dense.) But the salt gradient is destabilising: the saltier water, which we'd normally expect to be denser and therefore heavier, is above the fresh water.
"In the second case, which can occur in cold oceans, such as the Arctic, the gradients are reversed, with the colder, less salty water at the top and the warmer, saltier water at the bottom" says Hughes. (In this second case the salt gradient is stabilising and the thermal gradient is destabilising.) "In both cases, even though the overall density increases with depth, a doubly-diffusive instability can occur which can lead to staircase formation."
This matters as the development of such layered staircase formations affects the transport of heat and salt in the ocean. "The transport of heat (and salt) is very different in layered and non-layered states," says Hughes. "Understanding the layering process is critical to understanding the heat transport under sea ice, which itself is crucial to any predictions of how the ice may melt." But how these staircases form, how stable they are, and how they will be affected by climate change, is not well understood. "That's why we came together for six months to study them. And it's not just in fluid dynamics – anti-diffusion occurs in a whole range of physical and biological systems."
Mixing fields

Bird flocking is thought to be an example of another type of anti-diffusive process, which might occur when the birds go from flying independently, each moving through the sky in their own directions, to suddenly coalescing into one coherent flock moving together.
One approach to mathematically describing (modelling) how birds flock is to consider each individual bird like a separate particle, moving according to rules that depend on the position and movement of the birds around them. (You can see an example of how the flocking behaviour of birds is modelled this way in this article.) You can think of this as similar to a way we understand how magnets work, by thinking of them as an arrangement of magnetic atoms, whose alignment depends on the alignment of the atoms around them.
Another approach is to model the flock of birds as a whole, and instead mathematically describe the density of birds at any point in time and space. "And that is analogous to how we model fluids. We don't model every molecule, we average over everything to get the pressure or velocity at any point," says Hughes. This approach leads to the model of the bird flocking problem looking very similar to equations from fluid dynamics, albeit with some extra features.
"The link between the equations of fluid dynamics and flocking was probably well known, but not to me," says Hughes, who is himself from a fluid dynamics background. "This was the kind of thing that the programme was great for, I could understand what [researchers in that area] were doing."
Another type of anti-diffusive process is spinodal decomposition. This is when a consistent mixture of two substances unexpectedly separates out into clumps – something first observed in metal alloys containing different types of metal. The idea of spinodal decomposition was discussed during the ADI workshop on active matter, focussing on the behaviour of matter that has its own energy source, be it birds, bugs, or even something at a subcellular scale.
Joining the experts in fluid dynamics and active matter at the ADI programme were plasma physicists. They study the behaviour of plasma – the fourth state of matter (in addition to solids, liquids and gases) that is most commonly found in stars. Creating and controlling plasma is key to developing fusion reactors, where energy is created by fusing light elements together (mimicking what happens inside of stars) rather than splitting atoms apart (as is done in standard (fission) nuclear reactors). Fusion holds the promise of providing clean energy but we are still waiting for this technology to be viable.
Anti-diffusion might help meet one of the challenges of creating viable fusion reactors. The superhot plasma must be confined both to preserve enough heat to maintain the reaction, and also to protect the walls of the reactor. For example, tokamak reactors use a magnetic field to confine the plasma in a doughnut shape, the magnetic field winding around the doughnut. "One of the things that theoreticians and experimentalists have recognised is that a staircase can develop across the radius of the doughnut, and the staircase can have a big effect on this confinement," says Hughes. Understanding the anti-diffusive processes occurring inside the plasma within a fusion reactor might improve future designs.
"The interesting thing about the programme, and what the INI is fantastic for, is bringing together people who wouldn't normally meet at conferences," says Hughes. "You're exposed to problems you wouldn't normally hear about." And this opportunity to mix with people from different fields allows researchers to share knowledge and spot the commonalities. "It turns out that there are many common problems in anti-diffusive physics. You see the overlaps in different areas."
Bringing such diverse researchers together was an opportunity to work on a new mathematical foundation for understanding anti-diffusion. Can this help us understand ocean physics in order to help maintain the ice caps? Or might it help make fusion energy a reality? Ongoing collaborations between these diverse groups of researchers together might find the answers to these questions.
About this article
David Hughes is a professor in the School of Mathematics at the University of Leeds researching various aspects of astrophysical and geophysical fluid dynamics and magnetohydrodynamics. He was one of the organisers of Anti-diffusive dynamics: from sub-cellular to astrophysical scales, a six-month research programme at the Newton Institute for Mathematical Sciences in Cambridge in 2024.
Rachel Thomas is Editor of Plus.
This article forms part of our collaboration with the Isaac Newton Institute for Mathematical Sciences (INI) – you can find all the content from the collaboration here.
The INI is an international research centre and our neighbour here on the University of Cambridge's maths campus. It attracts leading mathematical scientists from all over the world, and is open to all. Visit www.newton.ac.uk to find out more.
