
Tracing mpox
Mpox has been spreading globally and it looks like it won't go away. WHO declared the current outbreak a public health emergency of international concern in July, and a recent report by the UK government says there's currently "no reason to assume that the epidemic as a whole will naturally reduce without interventions globally."
What we know about mpox
Mpox isn't a new virus. It has been endemic in several Central and West African countries for a while and we've previously had outbreaks in other parts of the world too. Despite the name of the disease, the virus' natural habitat appears to be in rodents, who can pass it on to humans. A small 2003 outbreak in the US appears to have come from a collection of squirrels, rats, porcupines and mice that had been shipped to Texas from Ghana and spread to humans via prairie dogs that were kept as pets. The reason it used to be called monkeypox is that monkeys were the species in which it was first discovered, in a lab in 1958.

Despite the name, the natural habitat of the mpox virus is in rodents. Image: Dave S., CC BY-SA 2.0.
Mpox is related to smallpox and can be severe or even fatal. Anyone can get it, but it doesn't spread anywhere near as easily as COVID-19 does. To catch it you need to come into close physical contact with an infected person, though there is also evidence that it can spread through towels, bedding or clothes. Symptoms include raised spots that turn into blisters, a high temperature, headaches, general aches, and so on — see the NHS website for more information.
The current UK outbreak ran to around 3000 confirmed cases as of 16 August 2022. The government's latest technical briefing found that the majority of cases are in networks of men who report themselves as gay, bisexual, or as men who have sex with men (GBMSM). Over 75% of all people diagnosed with mpox in this outbreak so far live in London and there appear to have been a few superspreading events, such as a festival that happened in Gran Canaria in June.
The good news is that we have an effective vaccine: the smallpox vaccine, which led to smallpox being declared eradicated in 1980, works for mpox too. In the UK it's currently offered to people that are considered at highest risk of catching the disease. That includes people who've had close contact with a confirmed case, healthcare workers looking after someone with mpox, and men from the GBMSM community if they're considered at high risk, for example because they have had multiple sexual partners. Because the supply of vaccine doses is limited, people who are not considered at a high risk currently aren't vaccinated.
What do we not know?
The short answer is "a lot". One glaring piece of ignorance is that we don't actually know the total number of cases in the UK. The 3000 quoted above refer to infections that have been confirmed by tests. "It's often the case in diseases like this that the cases you detect are far from all of the cases," says Jessica Enright, a disease modeller at the University of Glasgow. "There are also usually others that you don't detect for a variety of reasons. They might have no symptoms, or very mild symptoms — if you just have one or two spots you might not think anything of it. Or perhaps the cases are incorrectly identified."
.jpg)
Colorised transmission electron micrograph of mpox particles (teal) found within an infected cell (brown), cultured in the laboratory. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Image: NIAID, CC BY-SA 2.0.
Without knowing the total number of cases, it's difficult to estimate all those other quantities we've become familiar with through COVID-19. These include the famous R number, the growth rate of the of the epidemic, and more detailed information such as the probability that a certain kind of contact — a hug, a cuddle, or sex — leads to an infection. The latest government report puts the growth rate at 0 (encouraging news), but warns that this is just a rough estimate. "It's very uncertain because we are very likely missing lots of cases," says Enright. And if the case numbers are small, as they still are, then it is difficult to find statistical patterns.
Another thing that's hard to pin down is just how fatal mpox is. In Central Africa there's a clade (something akin to a family) of mpox with a reported death rate of up to 10%. "For the Western African version, which is the one that is more closely closer related to what we are seeing around the world right now, it is usually reported as somewhere around 1%," says Enright.
These percentages don't apply to the current outbreak in the UK, however. In the part of the world where mpox is not endemic the number of deaths is still low. There are five in total, two in Spain, one in India, one in Brazil, and one in Ecuador. There are various potential reasons for this difference between places where the disease is endemic and places where it's not. One is that healthcare systems differ. Another may be that in places like the UK the disease does not yet affect the very young or very old, who tend to be most vulnerable. And a third is what we already mentioned above: if the total number of cases is not known, then it's impossible to accurately estimate the death rate. Countries that don't have the resources to detect cases and monitor them therefore can't produce reliable estimates.
Tracing contact networks
One very important tool for discovering new cases, as well as slowing the spread, is contact tracing. Of course this doesn't guarantee you find everyone who is infected. Not everybody will know who their contacts were or be willing to reveal them, and not everybody will respond when they are traced because someone has named them as contact. An advantage of contract tracing, though, is that it reveals how infections are linked up: it reveals networks of infection.
"[This can give you] an indication that you are missing cases," says Enright. "If you are doing contract tracing when you detect cases, and find that most cases are not connected — so you get one case here, two joined-up cases there, maybe three over there — then you're probably missing a bunch of cases that join them together."
The networked nature of an epidemic also gives an in-road to modelling it. The general idea of network models (which were also used for modelling COVID-19) is to create a virtual network whose overall structure reflects the contact patterns of the population you are interested in. If you know the vital epidemiological parameters of a disease, such as the probability that a certain type of contact leads to the infection being passed on, then you can use your network structure to simulate the spread of the disease through the population.
You can also test what the effect of an intervention might be: people reducing their contacts would correspond to eliminating links from the network, and people getting vaccinated would reduce the probability of transmission. The effect of contract tracing itself can also be simulated as it leads to a proportion of the people who have been traced isolating and/or getting vaccinated.
Multi-layer networks
Enright, together with researcher Aeron Sanchez (based at the Roslin Institute, University of Edinburgh), is currently working on a network model for mpox, but the approach is cleverer than what we just described. It takes account of the fact that an individual is usually a member of several networks that might each come with their own structural characteristics. In the model, this is reflected by there being several layers of networks. A single node, representing a person, can be part of several of those layers.
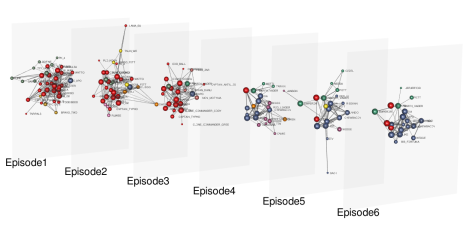
This is an example of a multi-layer network. It's not for mpox though, but for Star Wars: each layer corresponds to an episode and two nodes are connected each other if the corresponding characters acted together in one or more scenes. Image: Dedalus1234, CC BY-SA 4.0.
"In one layer we have the GBMSM sexual contact network," explains Enright. "Every node represents a person and there is a link between nodes if the people they represent have had sexual contact. I try and make this network look like what we know about the real [sexual contact network of the GBMSM community]."
To be clear, the nodes don't represent real individuals. Instead, we're looking at a hypothetical population of virtual people whose contact patterns reflect what is known about the GBMSM community. For example they reflect that the number of sexual contacts can differ wildly from person to person. This kind of information comes from sources like the National Survey of Sexual Attitudes and Lifestyle.
"Then there is another layer that [represents] household contacts, which are also important in mpox because there are lots of close contacts within households. Now we have a wider population that includes the population in the GBMSM layer." Here, small clusters of nodes that are all linked represent a household, and the distribution of sizes of these households represents what we know about the real population.
On top of these two layers you can also add other layers you think might be important, for example a layer reflecting the general population, or a layer for nurseries, or sports teams, and so on. Information on what the network of a layer looks like comes from contact surveys, such as POLYMOD.
If you have estimates of the important disease parameters — such as probabilities of transmission, the incubation period, the probability someone gets tested, etc — you can now run a simulation of the spread of the disease on the multi-layer network and also test the likely effect of interventions.
"The benefit of having separate layers is that you can include the characteristics of the network appropriate to the layer," explains Enright. "For example, contract tracing in the household layer is likely to be very successful because most people know who they live with and are willing to report that information. But it might not be very successful in the GBMSM sexual contact layer because people either can't or are uncomfortable reporting their sexual contacts. The model can take this difference into account."
Network detectives
The catch with what we have just described is that, at this moment in time, we do not have good estimates of important disease parameters. The good thing about networks, though, is that the exact details of which node is connected to which are not that important. What matters more is the overall shape of a network — whether it is made out of one giant connected component, or of lots of smaller connected components with a certain distribution of sizes, for example.
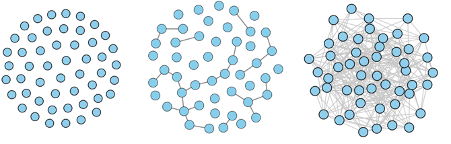
These three network have very different structures. The one on the left contains no links at all. The one in the middle contains several connected components. And the one on the right is made up of one giant connected component. The image appeared in our article on household bubbles relating to COVID-19 and originally appeared in Kawamoto et. al. 2015 – CC BY 4.0.
This means that even when there's uncertainty about parameters, network models can still provide useful information. If, for example, you know that a parameter lies somewhere in a given range, then you can run the model for lots of parameter values in that range. If the networks of infections that emerge from these simulations all have a similar overall shape, then that's evidence that the real network also has that kind of overall shape.
Enright is planning to use this approach, again with a focus on contact tracing. In the real world it's impossible to know how successful efforts to trace contacts are: do they on average find 90% of people who have had contact with a given infected person, or perhaps only 20%? Her model allows her to simulate the epidemic for various levels of contact tracing success. If the network of infections revealed by a particular setting of success in the model looks similar to what we see in real life, then that tells us that the real level of contract tracing success is probably roughly similar to the one used in the simulation.
The model can also suggest how successful contract tracing needs to be in each layer to discover the entire network of infection. "We can then talk to the people who do the contact tracing, to see if that level of success is even possible to achieve in practice. If not, then that's worth knowing. It means that we need to think about what resources we need to put into contact tracing to make it useful, or what other things we can do in addition."
What we have just described is still work in progress. But while Enright, along with a host of other disease modellers, is refining her model, statisticians and public health experts are working hard to refine estimates of those important parameters. Once good estimates are available, the models will be ready to go, providing projections that tell us just how worried we need to be, and what we can do to slow the spread of mpox.
In the meantime, it's important to remember that mpox isn't anywhere near as dangerous as COVID-19. "Everyone still has COVID on the brain for good reasons, there's a lot of it around," says Enright. "But in terms of transmissibility, we're really talking about totally different ballparks. If you're successfully protecting yourself from COVID, then you're probably successfully protecting yourself from mpox."
About this article
Marianne Freiberger, Editor of Plus, interviewed Jessica Enright in August 2022.
This article is part of our collaboration with JUNIPER, the Joint UNIversity Pandemic and Epidemic Response modelling consortium. JUNIPER comprises academics from the universities of Cambridge, Warwick, Bristol, Exeter, Oxford, Manchester, and Lancaster, who are using a range of mathematical and statistical techniques to address pressing questions about the control of COVID-19. You can see more content produced with JUNIPER here.
